Monoclonal antibody nomenclature
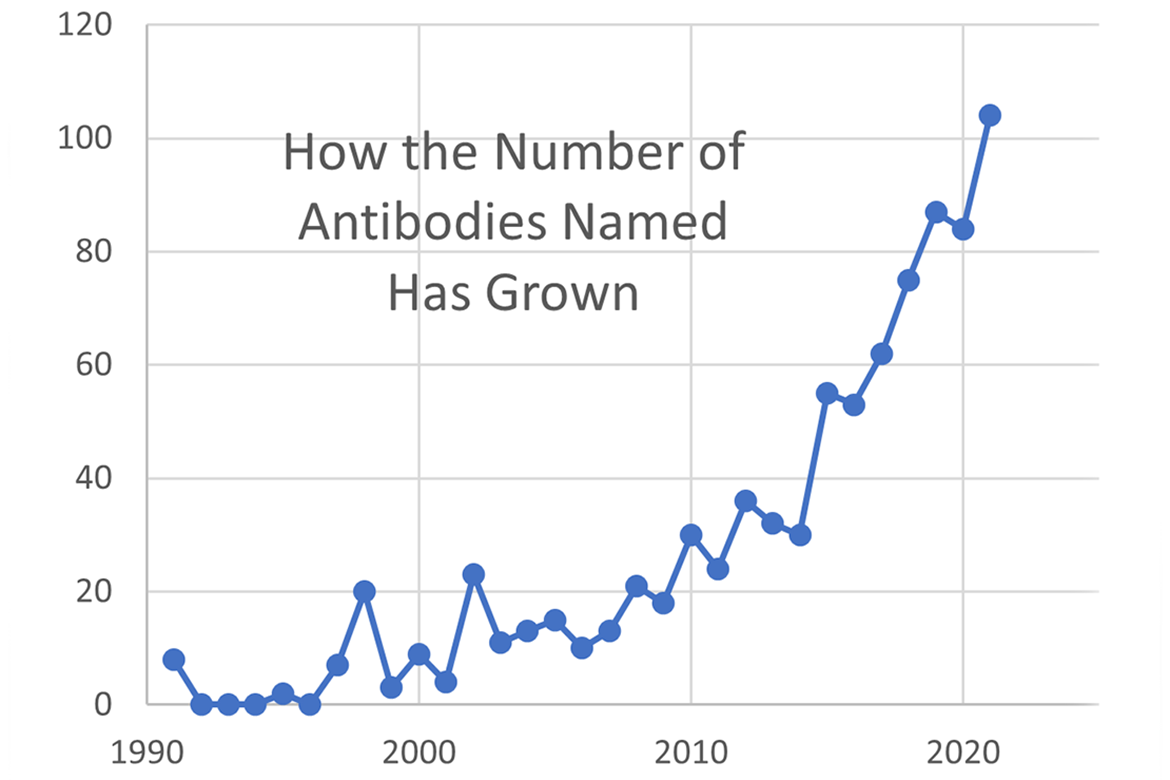
Recognizing the need for continued revisions because of crowding in the -mab stem class (over 800 monoclonal antibody names have now been selected), the USAN Council and INN Experts have revised the nomenclature scheme for monoclonal antibodies. USAN Program staff, USAN Council members and the FDA participated in developing the revisions to monoclonal antibody nomenclature published by INN.
Effective December 2021, the monoclonal antibody nomenclature scheme below is being used by both the USAN Council and INN Experts to name all new monoclonal antibodies that do not already have a USAN or INN.
The INN has published the monoclonal antibody nomenclature scheme (PDF).
Names that have already been adopted as USAN but not published on pINN or rINN lists will not be retroactively changed, nor will the USANC revise published INNs to apply the December 2021 version of the scheme.
If you have questions about a specific USAN negotiation that is a monoclonal antibody, please feel free to reach out to USAN staff and include your USAN file number in the correspondence.
The monoclonal antibody nomenclature scheme has changed several times since USAN began assigning names to monoclonal antibodies. Previous versions of the scheme are available (PDF).
Monoclonal antibody nomenclature scheme (effective December 2021)
Suffix. To increase variation in the suffixes of monoclonal antibody names, antibodies were divided into four groups.
Group 1 -tug
Unmodified immunoglobulins. Monospecific full length and Fc unmodified immunoglobulins of any class, as they might occur in the immune system, including:
- IgG, IgA, IgM, IgD, IgE
- only allelic variants
- Glycoengineering without mutation
- C-terminal lysine deletion without any other mutation in the Fc region
Group 2 -bart
Engineered or “artificial” immunoglobulins. Monospecific full immunoglobulins with engineered constant domains (CH1/2/3), monospecific full-length immunoglobulins containing any point mutation introduced by engineering for any reason anywhere (hinge, new glycan attachment site, mixed allelic variants not occurring in nature, altered complement binding, altered FcRn binding, altered Fc-gamma receptor binding, etc.)
Group 3 -mig
Multi-immunoglobulins. Bi- and multi-specific immunoglobulins of any format, type or shape (full length, full length plus, fragments)
Group 4 -ment
Fragments. Monospecific domains and fragments of any kind, derived from an immunoglobulin variable domain (all monospecific constructs that do not contain an Fc domain)
Infix. Infixes to denote the action or target of monoclonal antibodies will still be used. The list of available infixes for naming new antibodies is shown below. Please note that some infixes, most notably the -li- for immunomodulatory antibodies, has been discontinued. Others, most notably -ki- have new definitions.
Infixes to denote action or target of antibodies
Infix
Action or intended target
-ami-
serum amyloid protein (SAP)/amyloidosis
-ba-
bacteria
-ci-
cardiovascular
-de-
metabolic or endocrine pathways
-eni-
enzyme inhibitor
-fung-
fungal
-gro-
skeletal muscle mass related growth factors and receptors
-ki-
cytokines and cytokine receptors (Note: -ki- formerly defined for interleukins and interleukin receptors)
-ler-
anti-allergens
-ne-
neurologic indications
-os-
bone
-pru-
immunosuppressive
-sto-
immunostimulatory Note: includes checkpoint inhibitors
-ta-
tumors/oncology (not checkpoint inhibitors)
-toxa-
toxin
-vet-
veterinary use
-vi-
antiviral
Prefix. To create a unique name, a distinct, compatible syllable or syllables should be selected as the prefix. Suggested prefixes should comply with the USAN Program's rules for coining names. In addition, we ask that manufacturers avoid potential conflicts with names of other monoclonal antibodies. We expect, however, that implementing the new suffixes will reduce the number of conflicts with other names and make shorter prefixes possible.
Naming antibody-drug conjugates
Firms seeking to name an antibody-drug conjugate should file separate applications for the antibody with no payload (Form F), the payload alone (Form B) and the antibody and payload together (Form F). If a USAN has already been obtained for one or more of these substances, a USAN application is needed only for the substance for which a new name is required. This policy took effect on January 1, 2019, when the USAN Modified submission became applicable only for salts or esters of substance that have already received a USAN. For more information about how to apply, please see our USAN application forms page.
The USAN Program is often asked which form to fill out in specific situations.
- For a small molecule and its salt or ester, please use form A.
- For all substances for which there is a DNA, RNA or amino acid sequence, please use form F. When more than one name is requested, a separate form F should be filled out for each substance. Therefore, for example, an antisense oligonucleotide and its salt, or an antibody and an antibody-drug conjugate would require two applications using form F.
- Firms needing to revise the chemical, company, indication or other information associated with a substance should use form D.
- For a second name for the salt or ester of a substance that already has a USAN (or for which a USAN has been requested), form C should be used.
- For contact lens polymers, form E should be used.
Other modifications of USAN for monoclonal antibodies
Sometimes the name of a monoclonal antibody requires additional clarifying words.
If the antibody is conjugated to a payload, such as radiolabel or toxin, this conjugate is identified by using a separate, second word or other acceptable chemical designation. For antibodies conjugated to a toxin, the "-tox" stem must be included as part of the name selected for the toxin (e.g., zolimomab aritox, in which aritox identifies ricin A-chain). In other cases (e.g., brentuximab vedotin), the payload may receive a name based on a stem or a chemical name.
For radiolabeled products, the word order is:
- Name of the isotope
- Element symbol
- Isotope number
- Name of the monoclonal antibody, as follows:
- technetium Tc 99m biciromab
- indium In 111 altumomab pentetate
The “peg-“ prefix may be used for pegylated mAbs, but it should be avoided if it leads to an overly long name. Usually a 2-word name is preferable with the first word referring to the monoclonal antibody and "pegol" as the second word.
Unlike the INN Secretariat, the USAN Program does not append Greek letters (beta, gamma, etc.) to monoclonal antibodies to indicate new glycosylation patterns.
Sometimes, multiple monoclonal antibodies are combined in “cocktails.” For these substances, each monoclonal antibody receives a separate USAN/INN name.
For polyclonal mixtures of antibodies, "-pab" has been used. The -pab suffix applies to polyclonal pools of recombinant monoclonal antibodies, as opposed to polyclonal antibody preparations isolated from blood. This differentiates polyclonal antibodies from individual monoclonal antibodies named with -mab. Polyclonal antibodies are mixtures and therefore not named by INN.