Every year, millions of Americans are diagnosed with human immunodeficiency virus (HIV), sexually transmitted infections (STIs), viral hepatitis or tuberculosis (TB) and tens of thousands die from their infection. Guideline adherent screening programs can effectively identify these infections creating opportunities for treatment and prevention. Most of these infections share commonalities, from modes of transmission to demographic, social, and economic conditions that increase risk.
Routine screening and early detection of HIV, STIs, viral hepatitis and LTBI are critical for the health of our communities as these infections can have an asymptomatic stage which may prevent individuals from seeking the testing needed. For example, not everyone infected with TB bacteria becomes sick. As a result, two TB-related conditions exist: latent TB infection (LTBI) and TB disease. Persons unaware of their health status cannot take advantage of the treatment needed to improve their health and can unknowingly transmit the virus to other individuals.
The American Medical Association's (AMA) HIV, STIs, Viral Hepatitis and LTBI Routine Screening Toolkit for Community Health Centers and Emergency Departments is specifically designed to help clinicians overcome barriers and integrate screening into routine patient care.
The free toolkit (PDF) was vetted and reviewed by clinicians and physicians in community health centers and emergency departments nationwide, with a particular focus on practice leaders and champions who are looking to optimize routine screening practices. Its evidence-based approach walks through detailed, actionable steps for implementing routine screening to improve patient health outcomes.
What is routine screening?
By routine screening, we mean implementation of the Centers for Disease Control and Prevention (CDC) and U.S. Preventive Services Task Force (USPSTF) evidence-based preventive service guidelines and recommendations, including testing patients without symptoms based on patient characteristics or reported behaviors that would indicate screening is recommended. The AMA, as reflected in policy, supports the adoption and implementation of evidence-based preventive screening guidelines.
Implementing an effective routine screening program
Several critical considerations need to be understood and addressed to achieve successful routine screening practices.
- Infectious disease testing is often considered separate from routine care. Community health centers serve a variety of health needs. While screenings for blood pressure and cholesterol may be considered routine, screenings for infectious diseases may not. Infectious disease testing separate from regular routine care may reduce the likelihood people receive needed tests.
- Care is often complaint-based. A patient’s most immediate complaints often drive appointments, due to pressures of time and patient satisfaction. Frequently, routine infectious disease screening is not front of mind unless their complaint is related to symptoms of HIV, STIs, viral hepatitis or LTBI.
- Confusion over the definition of “routine” prevails. Clinicians often undertake testing only when a patient presents with certain risk factors associated with HIV, STIs, viral hepatitis or LTBI. While these risk factors may be included in routine screening guidelines, others in need of routine screening are often overlooked, and not everyone recommended for routine screening is tested. Uncertainty or discomfort over how to link persons diagnosed with HIV and other conditions to care may also discourage clinicians from screening.
- Stigma and fear persist. HIV, STIs, viral hepatitis and LTBI carry stigmas, and screening for these conditions comes with a fear of knowing one’s status—particularly with HIV. Clinicians sometimes need help working with patients to normalize screening and results counseling.
How to use the routine screening toolkit
The toolkit resources include a mix of both implementation and training-related materials for the care team. It is flexible, allowing you to follow along throughout the continuum to help improve your overall screening and testing approach or narrow in and focus on a specific stage. While not a specific stage, monitoring and evaluation along the screening continuum is critical and performance outcomes should inform continuous quality improvement efforts.
Given the specific populations recommended by CDC and USPSTF for LTBI screening in primary care settings, LTBI screening is not included in the routine screening strategies for emergency departments. Emergency departments should continue to consider TB disease in patients with risk factors or positive screening and diagnostic evaluations. For emergency departments who have strong active linkage to care systems established to support LTBI treatment, including staff dedicated to following up with the patient and facilitating confirmed linkage to treatment, and serve a patient population for whom LTBI screening is recommended, please refer to the community health center strategies for LTBI screening and treatment resources. Learn more on routine screening, STD and STI testing.
Access the community health center and emergency department toolkit strategies
Download the full toolkit
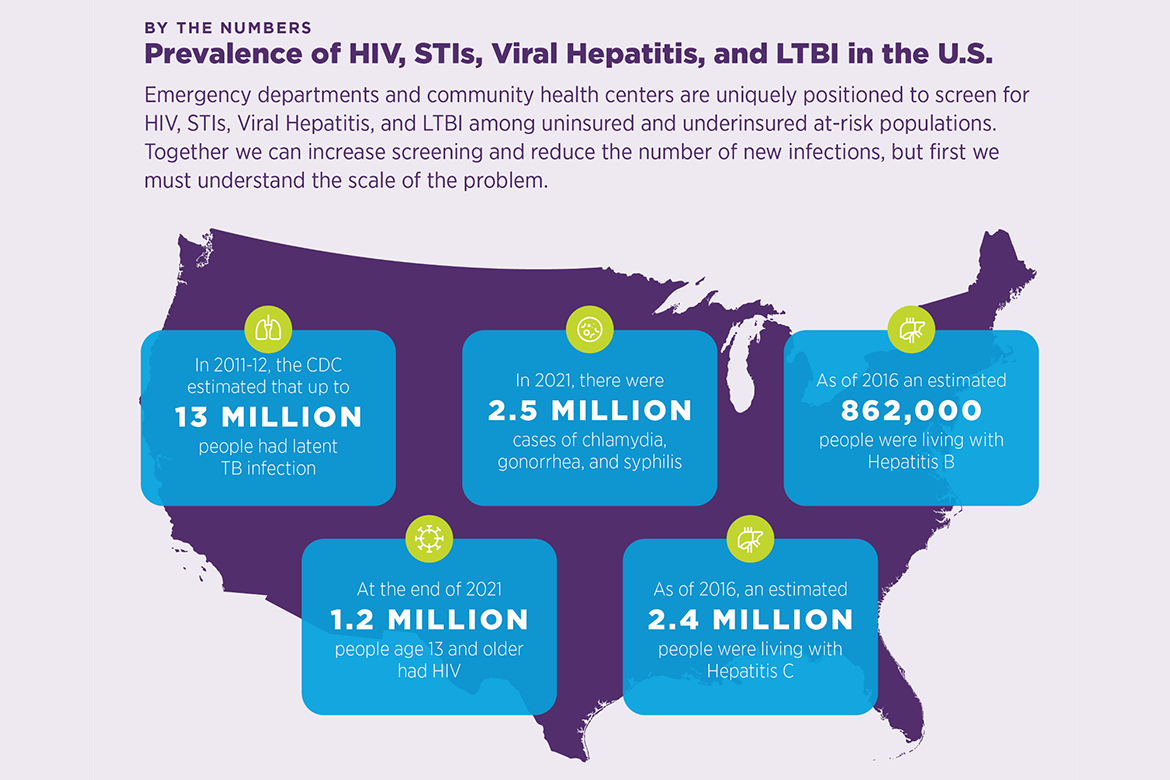
Impact by the numbers
Community health centers and emergency departments are uniquely positioned to screen for HIV, STIs, viral hepatitis, and LTBI among under-resourced and disproportionately affected populations. Together we can increase screening and reduce the number of new infections, but first we must understand the scale of the problem.
This effort is supported through a cooperative agreement with the Centers for Disease Control and Prevention’s (CDC) National Center for HIV, Viral Hepatitis, STD, and TB Prevention (CDC-RFA-OT18-1802: Strengthening Public Health Systems and Services through National Partnerships to Improve and Protect the Nation’s Health).